Experimental and theoretical study of Pb⋯S and Pb⋯O σ-hole interactions in the crystal structures of Pb(ii) complexes†
Abstract
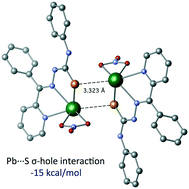
We report here the synthesis of two new Pb(II) compounds in which the lead center is coordinated by organic ligands via S and O donor atoms. Remarkably, in both compounds the Pb coordination is hemidirectional, which facilitates the approach of extra donors to establish interactions at longer distances. Such interactions are of σ-hole nature between the Pb and O/S atoms, acting as Lewis acid and bases, respectively. Interestingly, the Pb⋯S/O distances are closer to the sum of the covalent radii than to the van der Waals ones, which suggests a considerably strong interaction. We have performed a structural analysis of the crystal structures as well as a theoretical analysis based on DFT calculations to gain deeper insight into the origin and features of these σ-hole interactions. Moreover, the nature of the Pb⋯S/O interactions have been further analysed by means of AIM, MEP and NBO calculations.
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions


 Please wait while we load your content...
Please wait while we load your content...