Noncovalent interactions in the design of bis-azo dyes†
Abstract
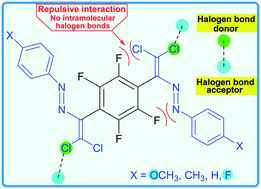
A series of new (1E,1′E)-2,2′-((perfluoro-1,4-phenylene)bis(2,2-dichloroethene-1,1-diyl))bis(1-(para-substituted phenyl)diazenes) have been synthesized via CuCl catalyzed olefination of the corresponding Schiff bases with CCl4 in the presence of tetramethylethylenediamine (TMEDA) in DMSO. Cl⋯O and Cl⋯F types of halogen bonds were found in these bis-azo dyes, which depend on the electron-donating or -accepting properties of the para-substituent (–OCH3, –CH3, –H, –F) of the azoaromatic ring. DFT calculations demonstrate that noncovalent interactions play a significant role in the stabilization of the intermolecular networks of the structures under study.
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions


 Please wait while we load your content...
Please wait while we load your content...