An experimental study on polymorph control and continuous heterogeneous crystallization of carbamazepine†
Abstract
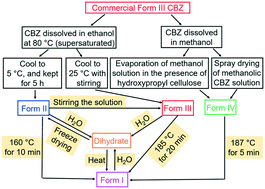
Forms I–III and dihydrate carbamazepine (CBZ) were prepared and confirmed by X-ray powder diffraction (XRPD) and differential scanning calorimetry (DSC). Influences of supersaturation (σ), stirring, anti-solvent (H2O), and polymer type on the resultant polymorph are discussed. For a CBZ ethanol solution at 5 °C, more than 10 h was required to form crystals when σ was 0.5, while less than 2 s was required when σ was increased to 9.0. Very fine needle-shaped Form II crystals were obtained when σ ≥ 7.5. Higher stirring rates facilitated the formation of Form III CBZ. Continuous heterogeneous crystallization of Form III on polyvinyl alcohol (PVA, MW 89 000–98 000) was achieved in a one-stage mixed suspension mixed product removal (MSMPR) crystallizer at 15 °C and 300 rpm. At 5 °C and 40 rpm, only Form II crystals were obtained. However, Form II CBZ gradually transformed to Form III within 2 residence times, and the transition process was irreversible.


 Please wait while we load your content...
Please wait while we load your content...
