Experimental and computational approaches to produce and characterise isostructural solvates†
Abstract
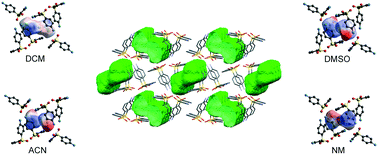
Dapsone (DDS) shows a rich solid form landscape, comprising five anhydrates, one hydrate and 15 solvates. The four isostructural DDS hemisolvates, with dichloromethane, acetonitrile, nitromethane and dimethyl sulfoxide as guest molecules, obtained by cooling crystallisation, slurry or solvent vapour diffusion experiments were investigated in this study. Experimental (thermal analysis and X-ray diffraction) and computational studies (lattice, intermolecular and stability energy calculations) were undertaken to rationalise and understand (hemi)solvate formation, stability and phase transitions occurring during the desolvation of the solvates. It was found that DDS⋯solvent interactions and the shape/size of the solvent molecules are critical for solvate formation. Careful control of the experimental crystallisation and storage conditions were required for identifying and characterising solvates that are unstable at ambient conditions. The solvent molecules of the hemisolvates are located at isolated-sites and form strong hydrogen bonds and/or interactions involving dispersion forces. The isostructurality of the four hemisolvates was confirmed by single crystal X-ray diffraction determinations and modelling, i.e. solvent exchange calculations. Furthermore, the formation of either the hemi- or monosolvate stoichiometry was rationalised on the basis of lattice energy calculations.


 Please wait while we load your content...
Please wait while we load your content...