Drug–drug salts of mefenamic acid\tolfenamic acid and piperazine to improve physicochemical properties for potential veterinary use†
Abstract
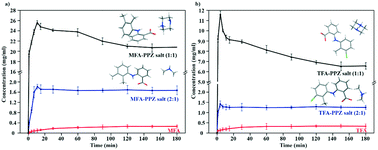
The incorporation of drug–drug salts is known as an attractive strategy to not only improve physicochemical properties but also extend the field of combination drug delivery. Mefenamic acid (MFA) and tolfenamic acid (TFA) belong to effective but poorly soluble anti-inflammatory drugs used for treating both humans and animals. Piperazine (PPZ) has anthelmintic activities with a serious hygroscopic problem. To improve the physicochemical properties of such drugs and seek possible drug combinations, herein, four novel MFA\TFA–piperazine (PPZ) drug–drug salts with different stoichiometry (2 : 1 and 1 : 1) were designed and synthesized. As we expected, the experiments proved that their solubilities and dissolution properties were much better than that of MFA and TFA. Among them, the apparent solubility of the MFA–PPZ salt (1 : 1) improved about 130 times more than that of MFA in a mixture of water and ethanol. Meanwhile, these salts were non-hygroscopic based on dynamic vapor sorption analysis. Moreover, the obtained crystals were fully analyzed by single-crystal X-ray diffraction (SXRD), Hirshfeld surface analysis and a solvent mediated phase transformation study, which provided a better understanding of the crystal structure. The MFA–PPZ salt (2 : 1) and TFA–PPZ salt (2 : 1) possessed a similar crystal structure and their solubilities were also similar. In addition, the incorporation of PPZ in the crystal lattice destroyed the dimer form in the original MFA\TFA crystal and resulted in the formation of salts through a new ionic hydrogen bond. After contact with the solvents, PPZ was more likely to interact with the solvent molecules, which may have led to the acceleration of dissolution.


 Please wait while we load your content...
Please wait while we load your content...