Synthesis of nanocrystalline strontium titanate by a sol–gel assisted solid phase method and its formation mechanism and photocatalytic activity
Abstract
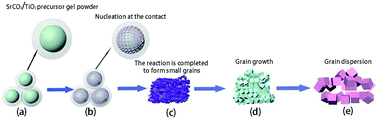
Strontium titanate (SrTiO3) with a perovskite structure is widely applied to hydrogen production by photolysis water splitting. However, the existing preparation techniques cannot make uniform high-purity strontium titanate without causing pollution under simple conditions. Therefore, a preparation technique that can mass-produce uniform high-purity and environmentally friendly strontium titanate at low temperatures is particularly important. In this paper, a sol–gel method is used to coat strontium carbonate with a TiO2 precursor. The core–shell structure formed by this structure increases the contact area between the reactants, which is beneficial to the diffusion between elements and promotes the kinetic process of the reaction, and pure-phase nano-strontium titanate can be made at low temperatures. The microstructure of the samples before and after calcination at 800 °C is analyzed by FT-IR. The chemical composition and the valence state of the calcined samples at 800 °C are analyzed by XPS. The structure of the samples at different heat treatment temperatures is observed and the structure of the samples at the same heat treatment temperatures with different heat treatment times is observed by XRD and transmission electron microscopy. TGA-DSC is used to monitor the changes in mass and heat during the reaction. The transformation mechanism and nucleation process of the material are analyzed from the perspective of thermodynamics and kinetics. In addition, the UV-visible absorption spectrum is used to calculate the forbidden band width. Finally, the activity of the hydrogen produced by water-splitting the sample under different heat treatment conditions is measured. The results showed that strontium titanate was produced at the contact interface at 600 °C, and the pure phase of strontium titanate was produced at 800 °C with a size of 34 nm. The reaction process conforms to the nucleation-growth model and the grain grows with the driving force of grain boundary and the temperature increase. The forbidden band width of the pure-phase strontium titanate produced after calcination at 800 °C is 3.173 eV, with the best photocatalytic performance, and its hydrogen production rate is 2.40 μmol g−1 mol−1. This work provides a new preparation process for nano-strontium titanate and may be highly applied to hydrogen production by photolysis water splitting.


 Please wait while we load your content...
Please wait while we load your content...