Transfer of chirality in N-triphenylacetylamino acids and chiral derivatives of N-triphenylacetyl Gly–Gly dipeptide and control of their assembly with steric constraints†
Abstract
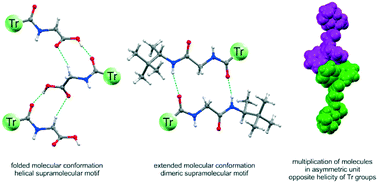
The aim of this work was to show the various aspects associated with incorporation of a sterically demanding trityl (CPh3, Tr) group into a series of N-acylated amino acids, N-acylated Gly amides and chiral Gly–Gly dipeptide derivatives. Structural analysis, carried out with the use of complementary experimental methods and supported by DFT calculations, enabled us to determine the mechanism of chirality transmission from the stereogenic center to the trityl reporter group. The sensitivity in chirogenesis is a function of steric requirements of the amino acid backbone, the inductor–reporter distance and the environment polarity. Crystal structures were analyzed as a function of the size and character of the substituent at the Cα carbon and in relation to the Tr moiety as a supramolecular protective group for the N–H hydrogen-bond functionality. We have demonstrated that back-activation of the N–H group is possible by incorporation of additional functional groups that display a strong hydrogen bond accepting ability, but this process is inevitably connected with the structural response of the Tr group in the form of severe deformation of its propeller shape. The study has revealed a pivotal role played by the N-triphenylacetyl group in reducing the dimensionality of supramolecular aggregates as compared to the N-acetyl substituent.


 Please wait while we load your content...
Please wait while we load your content...