Towards the synthesis of mixed oxides with controlled stoichiometry from Prussian blue analogues†
Abstract
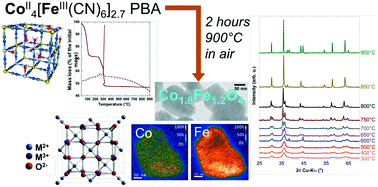
Controlling the synthesis of mixed oxides is of great interest since their stoichiometry influences the properties. We present here the transformation of the Co4[Fe(CN)6]2.7 Prussian blue analogue (PBA) into the mixed Co1.8Fe1.2O4 spinel oxide, with a detailed characterization of the final phase (obtained by calcination in air at 900 °C for 2 hours) and an emphasis on the calcination process by TDA/TGA, X-ray diffraction, (high-resolution) TEM and energy-filtered TEM. Single-crystalline particles with a homogeneous distribution of Fe and Co ions within each particle are observed, with the same Co : Fe ratio as in the PBA. The calcination mechanism is a 2-step transformation, with first the elimination of water molecules and then the cyanide decomposition. XRD and TEM at intermediate temperatures additionally show the occurrence of a phase separation between 500 °C and 850 °C before the formation of the single final phase. We demonstrate also through the calcination of intermediate PBAs between Co4[Fe(CN)6]2.7 and Co4[Co(CN)6]2.7 that this synthesis route leads to the perfect control of the oxide stoichiometry through control of the PBA. The versatility of this synthesis route is finally illustrated through the successful synthesis of Co1.8Fe1.2O4 nanoparticles (diameter ∼5 nm) from PBA nanoparticles confined within the pores of a mesoporous silica monolith.


 Please wait while we load your content...
Please wait while we load your content...