Structure and adhesion energy of the (10.4) calcite/(001) ice Ih and (210) baryte/(001) ice Ih interfaces†
Abstract
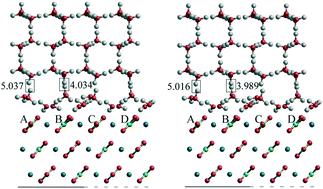
In this contribution we performed ab initio quantum-mechanical calculations to determine the equilibrium structures and specific adhesion energies (β) at 0 K of (10.4)-calcite/(001)-ice Ih and (210)-baryte/(001)-ice Ih interfaces: calcite (Cal), CaCO3 and baryte (Brt), BaSO4. Successively, by means of the calculated adhesion energies and some approximations, the specific interface energy (γ) at 273.15 K of both interfaces was estimated and compared, in the case of calcite, with the (10.4)-calcite/water interface energy at the same temperature. Moreover, we made some general considerations on the role of enthalpy and entropy to explain the hydrophilicity and hydrophobicity of the crystal surfaces. We found that ice is strongly bound to the (10.4)Cal and (210)Brt surfaces, with their adhesion energies being not negligible: βCal/Ice(10.4)/(001) = 0.240 and βBrt/Ice(210)/(001) = 0.336 J m−2. Moreover, we found that the interface energy at 273.15 K of the (10.4)Cal/(001)Ice system, γCal/Ice(10.4)/(001), is 0.333 J m−2, significantly lower than the interface energy of the (10.4)Cal/water system (γCal/W(10.4) = 0.412 J m−2) at the same temperature; the interface energy at 273.15 K of the (210)Brt/(001)Ice system, γBrt/Ice(210)/(001), goes down to 0.117 J m−2, a value extremely lower than that obtained for the (10.4)Cal/(001)Ice interface.


 Please wait while we load your content...
Please wait while we load your content...