Probing non-covalent interactions driving molecular assembly in organo-electronic building blocks†
Abstract
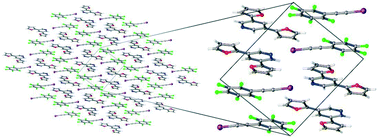
Recent advancements in material science exploit non-covalent interactions, such as halogen bonding (XB) or π-stacking within solid-state molecular frameworks for application in organic electronic devices. Herein, we focus on these and other non-covalent interactions and the effect that furan and thiophene substituents play on the solid-state properties of co-crystals formed between pentafluoro(iodoethynyl)benzene (F5BAI; XB donor) and a pyridine disubstituted with either furans or thiophenes (PyrFur2 and PyrThio2; XB acceptors). Spectroscopic and thermal analyses of 1 : 1 mixtures provide indirect evidence of XB interactions, whereas X-ray crystallography provides direct evidence that XB and π-stacking are present in both co-crystals. Density functional theory (DFT) computations provide insight into the relative electronic energetics of each pair-wise contact observed in the experimental F5BAI-PyrFur2 and F5BAI-PyrThio2 co-crystals.
- This article is part of the themed collection: Halogen Bonding in Crystal Engineering Editor’s collection


 Please wait while we load your content...
Please wait while we load your content...