Intermolecular head-to-head interaction of carbonyl groups in bicyclic hydrogen-bonded synthon based on β-hydroxy ketones†
Abstract
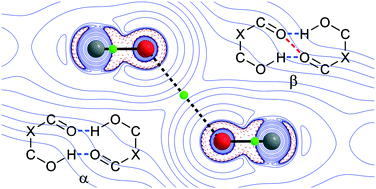
In this study, we report a counterintuitive carbonyl–carbonyl interaction explored for crystalline (2RS,3RS)-1-aryl-2-bromo-3-hydroxy-3-(2-nitrophenyl)-propan-1-ones. The mentioned β-hydroxyketones display an ability to form dimensionally different crystal formative motifs ranging from 1D to 3D. Despite the structural differences, primarily in intermolecular interactions, all the studied compounds are arranged by the same bicyclic hydrogen-bonded twelve-membered supramolecular synthon R22(12), with the essential feature being an attractive interaction between two oxygen atoms of the head-to-head-arranged carbonyl groups C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯O
O⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C. This unusual interaction along with all the other interactions that are present in the crystal structures, for instance, H-bonds and hydrogen–hydrogen and carbonyl–halogen contacts, were investigated by means of quantum-topological analysis of the calculated electron density.
C. This unusual interaction along with all the other interactions that are present in the crystal structures, for instance, H-bonds and hydrogen–hydrogen and carbonyl–halogen contacts, were investigated by means of quantum-topological analysis of the calculated electron density.


 Please wait while we load your content...
Please wait while we load your content...