Polymorphism and molecular conformations of nicosulfuron: structure, properties and desolvation process†
Abstract
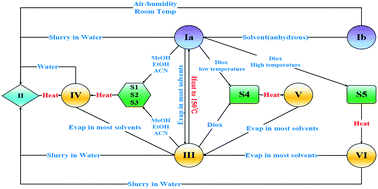
Four new solvent-free forms and five solvates of the widely-used herbicide nicosulfuron were found. The crystal structures of the reported form II and two new solvates (methanol and acetonitrile) were revealed for the first time. The results showed that nicosulfuron is conformationally flexible and that nine conformations are involved in these three solid forms, leading to different intermolecular interactions and packing patterns. The relationship between the polymorphs and molecular conformations was discussed through crystal structure analyses. In addition, comprehensive characterization of these forms was carried out to study the desolvation process and stability. The results showed that temperature-induced desolvation led to the appearance of new polymorphs, and that the moisture-dependent stability of nicosulfuron improved significantly compared to the marketed form Ia.


 Please wait while we load your content...
Please wait while we load your content...