Intramolecular geometry and intermolecular interactions of the CNO group of crystalline benzonitrile oxides: a comparison with phenyl cyanates, phenyl isocyanates, and phenyl azides†
Abstract
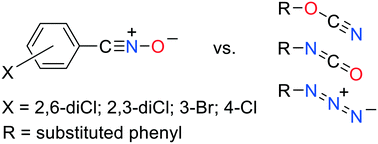
Determination of the crystal structures of 2,6-dichlorobenzonitrile oxide (2,6-diCl), 2,3-dichlorobenzonitrile oxide (2,3-diCl), 3-bromobenzonitrile oxide (3-Br), and 4-chlorobenzonitrile oxide (4-Cl) has allowed examination of the variation in the geometry of the nitrile oxide group as a function of ring substitution as well as comparison of the solid-state molecular packing motifs of these reactive compounds. The geometry of the CNO group is found to be essentially linear in sterically unhindered 4-Cl but bent by as much as eleven degrees at the Cring–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N angle in more hindered benzonitrile oxides reported by previous investigators. Comparison of the nitrile oxide group geometry with the geometries of the three-atom functional groups of phenyl cyanates, phenyl isocyanates, and phenyl azides shows that in spite of their greater flexibility, the degree of bending in even the most nonlinear benzonitrile oxides does not approach that in these other three pseudohalogens. This difference in geometry may prevent their isomorphism. Isomorphism even among the reported phenyl cyanates, phenyl isocyanates, and phenyl azides is rare, although the relatively few structures reported in the Cambridge Structural Database of particularly the cyanates and isocyanates, as well as of the nitrile oxides, offer only a limited basis for comparison. A search of those structures for two-point intermolecular contacts between the three-atom functional groups in all four chemical families has revealed some initial relationships among them and may serve as a basis for future investigation of their solid-state reactivity.
N angle in more hindered benzonitrile oxides reported by previous investigators. Comparison of the nitrile oxide group geometry with the geometries of the three-atom functional groups of phenyl cyanates, phenyl isocyanates, and phenyl azides shows that in spite of their greater flexibility, the degree of bending in even the most nonlinear benzonitrile oxides does not approach that in these other three pseudohalogens. This difference in geometry may prevent their isomorphism. Isomorphism even among the reported phenyl cyanates, phenyl isocyanates, and phenyl azides is rare, although the relatively few structures reported in the Cambridge Structural Database of particularly the cyanates and isocyanates, as well as of the nitrile oxides, offer only a limited basis for comparison. A search of those structures for two-point intermolecular contacts between the three-atom functional groups in all four chemical families has revealed some initial relationships among them and may serve as a basis for future investigation of their solid-state reactivity.


 Please wait while we load your content...
Please wait while we load your content...