Solid state photodimerization of 9-tert-butyl anthracene ester produces an exceptionally metastable polymorph according to first-principles calculations†
Abstract
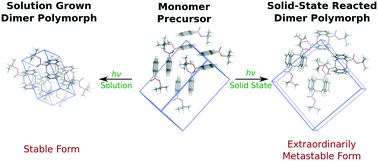
Molecular crystal engineering seeks to tune the material properties by controlling the crystal packing. However, the range of achievable properties is constrained by the limited energy range of polymorphs which can be crystallized. Here, computational modeling highlights that a solid-state crystal-to-crystal chemical reaction in 9-tert-butyl anthracene ester (9TBAE) nanorods [Al-Kaysi et al., J. Am. Chem. Soc., 2006, 128, 15938] imparts “synthetic memory” into the crystal structure that allows reproducible formation of a highly metastable, yet long-lived polymorph. Specifically, whereas the vast majority of known polymorphs exhibit lattice energy differences below 10 kJ mol−1, the conformational polymorph formed via solid state reaction chemistry lies 14 kJ mol−1 higher in energy than the form grown from solution, according to calculations that combine a dispersion-corrected second-order Møller–Plesset perturbation theory (MP2D) treatment of the monomer and photodimer with a density functional theory treatment (B86bPBE-XDM) of the intermolecular interactions in the crystal. Moreover, the solid-state reaction environment traps a highly unstable intramolecular photodimer conformation which defies the conventional wisdom surrounding conformational polymorphs. These observations suggest that solid-state reaction chemistry represents an under-appreciated strategy for producing polymorphs that would likely be unobtainable otherwise.
- This article is part of the themed collection: Editor’s Collection: Polymorphism in Molecular Crystals


 Please wait while we load your content...
Please wait while we load your content...