“Lp⋯synthon” interaction as a reason for the strong amplification of synthon-forming hydrogen bonds†
Abstract
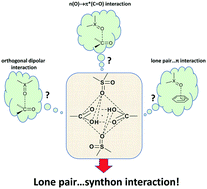
An original supramolecular motif has been found in one of two polymorphic modifications of (3,4-dichloro-2-oxo-2,5-dihydrofuran-5-ylsulfonyl)ethanoic acid: above and below the center of the classical carboxyl H-bonded dimer, the sulfonyl groups of two other molecules of the same compound are located. The interaction of the oxygen atoms of these sulfonyl groups with the carboxyl dimer synthon can be named the “lp(lone pair)⋯synthon”-type interaction. A strong amplification of the synthon-forming H-bonds is directly linked to the occurrence of such an interaction. The nature of the interactions is characterized by quantum-chemistry models using R. Bader's theory (QTAIM), the values of electrostatic potentials (MESP), and Pendás's approach based on interacting quantum fragments (IQFs).


 Please wait while we load your content...
Please wait while we load your content...