Recognition of the π-hole donor ability of iodopentafluorobenzene – a conventional σ-hole donor for crystal engineering involving halogen bonding†
Abstract
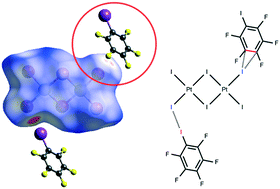
Iodopentafluorobenzene (IPFB or PhFI) was co-crystallized with tetra(n-butyl)ammonium tetraiodo-μ,μ′-diiododiplatinate(II), [n-Bu4N]2[Pt2(μ-I2)I4] (1), to give the adduct 1·2IPFB. The XRD experiment revealed that 1·2IPFB displays previously unreported C⋯I–Pt anion–π interactions formed along with the expected PhF–I⋯I–PtII halogen bond (XB); these two interactions join two complexes and two IPFBs in a heterotetrameric cluster. Processing of the available CSD data revealed only one structure (CSD code: IKIYAE) with a heterotetrameric cluster bearing simultaneous two PhF–I⋯X (X = I–PtIV, N) XBs and C⋯I lp(I)–π contacts between the two IPFBs. Results of the DFT calculations (M06/DZP-DKH level of theory) followed by the topological analysis of the electron density distribution within the framework of Bader's approach (QTAIM) for both 1·2IPFB and IKIYAE confirmed the availability of these (anion/lp)–π weak interactions. The estimated energies of the observed (anion/lp)–π and XBs contacts are in the 0.9–1.3 kcal mol−1 and 1.3–5.3 kcal mol−1 ranges, respectively. π-Hole donor ability of IPFB was additionally confirmed by theoretical calculations of the molecular surface electrostatic potential for the optimized equilibrium structure of IPFB.
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions


 Please wait while we load your content...
Please wait while we load your content...