Lone pair⋯π interaction versus σ-hole appearance in metal-bonded halogens†
Abstract
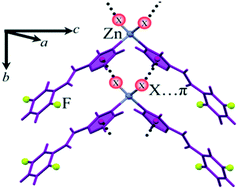
Three discrete complexes of ZnX2 and L2,5-F including [ZnCl2(L2,5-F)2], 1, [ZnBr2(L2,5-F)2], 2 and [ZnI2(L2,5-F)2], 3, in which L2,5-F is N-(2,5-difluorophenyl)-2-pyrazinecarboxamide, have been synthesized and characterized. The crystal structure data resulting from X-ray diffraction reveal the fundamental difference in the coordination sphere and supramolecular architecture of these compounds in comparison with homologous mercury complexes. Results clearly show that in all three coordination compounds, metal-bound halides have interacted with π systems through a negative electrostatic region. In addition, geometric parameters verify the lone pair⋯π nature of this halogen⋯π interaction. To provide clear insight, the NCI approach for these interactions has been performed. According to NCI analysis, halide-involved interactions, including Zn–X⋯πpyz and C–H⋯I–Zn, play a significant role in the supramolecular architecture of zinc(II) complexes and interactions of fluorine are of no importance in supramolecular assembly.
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions


 Please wait while we load your content...
Please wait while we load your content...