Fluconazolium oxalate: synthesis and structural characterization of a highly soluble crystalline form†
Abstract
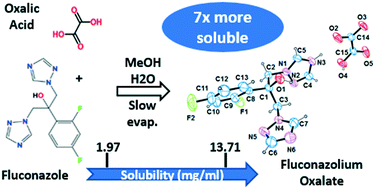
Fluconazole (FLZ) is one of the most used antifungal drugs worldwide and has been the focus of various investigations with the aim of enhancing its biomedical application. Most of the new solid forms achieved for this drug were determined by powder X-ray diffraction, and a few reports on polymorphs, solvates, cocrystals, and one salt–cocrystal by single-crystal X-ray diffraction (SCXRD) are available. By considering that FLZ is a very weak base (pKa = 1.76, when protonated), salt formation with this drug is expected with the use of very strong organic acids, in order to dislocate the proton from the acid to the FLZ. To the best of our knowledge, only one organic salt of FLZ with picric acid (pKa = 0.38) and one salt–cocrystal (maleate–maleic, pKa1 = 1.92) were reported by SCXRD until now. Having in mind all the advantages that pharmaceutical salts have in drug delivery, in this work we depict the methodology and techniques employed to synthesize a new salt of FLZ using oxalic acid (pKa1 = 1.23). The screening process was initially monitored using Raman spectroscopy, while further characterization by X-ray diffraction (powder and single crystal) and thermal analysis (DSC and TG) was performed to confirm the new salt fluconazolium oxalate (1 : 1). Finally, salt equilibrium solubility studies confirmed an improvement, about 7-fold, when compared to the commercialized active pharmaceutical ingredient (polymorph II). In addition, this is the first reported GRAS (generally regarded as safe) salt of the antifungal drug fluconazole.
- This article is part of the themed collection: Celebrating Latin American Talent in Chemistry


 Please wait while we load your content...
Please wait while we load your content...