Modulation of coordination in pincer-type isonicotinohydrazone Schiff base ligands by proton transfer†
Abstract
We present here two different coordination polyhedra of pincer type N2O hydrazone based ligands supplemented with thiocyanate ions. The compounds namely [Hg(SCN)2(HL1)] (1) and [Hg(SCN)2(HL2)] (2) have a common isonicotinohydrazone fragment and have been prepared by using a coordination driven self-assembly of the Hg(SCN)2 with two different ligands including 2-benzoylpyridine-isonicotinoylhydrazone (HL1), and 2-acetylpyridine-isonicotinoylhydrazone (HL2). In compound 1 the ligand coordinates to the mercury center in the keto form (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–NH
N–NH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
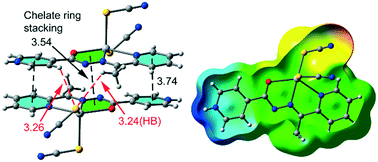
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) whereas, in compound 2, the proton at the hydrazine group has been shifted to the uncoordinated pyridine ring and the ligand acted as a zwitterion. The structures provide a complementary system for proton transfer within the ligand molecule involving the keto–enol tautomerization of the amide group and 4-pyridyl N protonation. As a result, the relative location of orbitals and ligands in the complexes are different as well as the bonding strength and the coordination polyhedra. We have also studied electrostatically enhanced π⋯π (either conventional or involving the chelate ring) interactions observed in the solid state of both compounds and analyzed them using DFT calculations, molecular electrostatic potential surface and Bader's theory of atoms in molecules.
O) whereas, in compound 2, the proton at the hydrazine group has been shifted to the uncoordinated pyridine ring and the ligand acted as a zwitterion. The structures provide a complementary system for proton transfer within the ligand molecule involving the keto–enol tautomerization of the amide group and 4-pyridyl N protonation. As a result, the relative location of orbitals and ligands in the complexes are different as well as the bonding strength and the coordination polyhedra. We have also studied electrostatically enhanced π⋯π (either conventional or involving the chelate ring) interactions observed in the solid state of both compounds and analyzed them using DFT calculations, molecular electrostatic potential surface and Bader's theory of atoms in molecules.


 Please wait while we load your content...
Please wait while we load your content...