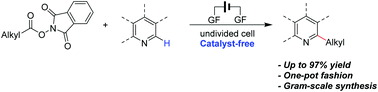
Catalyst-free electrochemical decarboxylative cross-coupling of N-hydroxyphthalimide esters and N-heteroarenes towards C(sp3)–C(sp2) bond formation†
Abstract
Cheap and widely available carboxylic acids are a class of ideal substrates to construct valuable compounds. As a candidate of decarboxylative reactions, the acid-based neutral N-hydroxyphthalimide ester undergoes a reductive decarboxylative process rather than a common oxidative decarboxylative process, which is a potential transformation mode for new reactions. In this work, we developed an electrochemical C(sp3)–C(sp2) coupling of N-hydroxyphthalimide esters and N-heteroarenes without any catalysts. Remarkably, this electrochemical protocol can not only be directly realised by carboxylic acids in a one-pot fashion, but also be scaled up using a continuous-flow reactor.


 Please wait while we load your content...
Please wait while we load your content...