De novo formation of citrate-based fluorophores on N-termini of peptides and proteins in cells and tissues†
Abstract
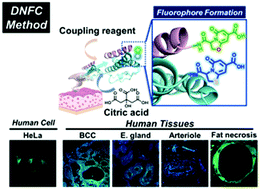
We developed a new method for the de novo formation of fluorophores based on citrate (DNFC) in biological samples. Use of an amide coupling reagent and microwave irradiation greatly facilitates the fluorophore formation on peptides and proteins with N-terminal cysteine or serine. Since N-terminal cysteine and serine can form thiazolopyridone- or oxazolopyridone-based fluorophores emitting blue and green fluorescence, respectively, by the DNFC staining, each organelle, cell and tissue exhibited a characteristic fluorescence distribution. The DNFC staining is able to provide a new potential protocol for future cell imaging, histology and diagnosis.


 Please wait while we load your content...
Please wait while we load your content...