Synthesis of fluorinated amphoteric organoborons via iodofluorination of alkynyl and alkenyl MIDA boronates†
Abstract
The iodofluorination of alkynyl and alkenyl MIDA (N-methyliminodiacetyl) boronates led to the synthesis of two types of fluorinated organoborons bearing a valuable C–I bond. The B(MIDA) moiety confers exclusive regioselectivity to the reaction, and the products were formed in generally good yields. Preliminary utility of the products was demonstrated.
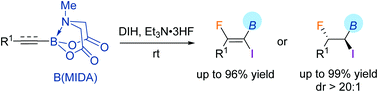


 Please wait while we load your content...
Please wait while we load your content...