Regioselective SNAr reaction of the phenoxathiin-based thiacalixarene as a route to a novel macrocyclic skeleton†
Abstract
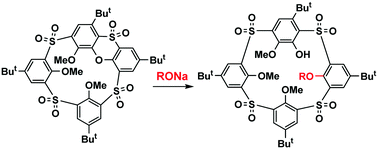
The sulfonyl analogue of phenoxathiin-based thiacalix[4]arene, easily accessible from the parent thiacalix[4]arene, reacts with sodium alkoxides to yield a cleaved product representing a novel type of macrocyclic skeleton with a quasi-calixarene structure. As shown by comparison with other derivatives, the internal strain imposed by the heterocyclic moiety is a driving force of this SNAr reaction.


 Please wait while we load your content...
Please wait while we load your content...