Convenient synthesis of phosphinecarboxamide and phosphinecarbothioamide by hydrophosphination of isocyanates and isothiocyanates†
Abstract
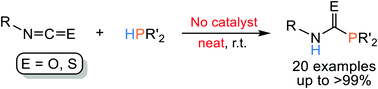
Reactions of isocyanates with primary and secondary phosphines without solvent at room temperature afforded the corresponding phosphinecarboxamide (RN(H)COPR′2) in excellent yields. This reaction system is applicable for isothiocyanates. The compounds newly obtained were fully characterized using multielement NMR spectroscopy. In addition, the molecular structure of Cl(CH2)2N(H)COPPh2 was studied by single-crystal X-ray diffraction.


 Please wait while we load your content...
Please wait while we load your content...