Pyridinium 1,4-zwitterionic thiolates as a useful class of sulfur-containing synthons: application to the synthesis of 2,5-dihydro-1,4,5-thiadiazepines†
Abstract
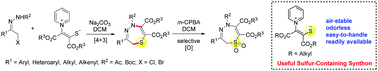
A novel [4+3] cascade cyclization reaction of pyridinium 1,4-zwitterionic thiolates with azoalkenes derived from α-halo hydrazones in situ has been developed. This reaction was found to allow expedient access to an array of 2,5-dihydro-1,4,5-thiadiazepines. Libraries of highly functionalized sulfoxide and sulfone analogues of 2,5-dihydro-1,4,5-thiadiazepines were also obtained via selective oxidation with m-CPBA.


 Please wait while we load your content...
Please wait while we load your content...