A coumarin chalcone ratiometric fluorescent probe for hydrazine based on deprotection, addition and subsequent cyclization mechanism†
Abstract
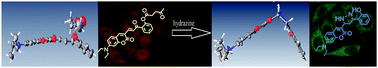
A ratiometric fluorescent probe for hydrazine based on a coumarin chalcone framework and a levulinic acid terminal group with a low detection limit (0.1 ppb, 0.003 μM), a large ratiometric fluorescence change (I465/I575, 1265-fold enhancement) and a wide pH work range (3.0–12.0) was developed. The mechanism analysis of the isolated hydrazine product characterized by NMR, HRMS and the crystal structure indicates that the levulinic acid group is firstly removed by deprotection and then the dihydropyrazole ring is formed due to the addition and subsequent cyclization reaction in the presence of hydrazine.


 Please wait while we load your content...
Please wait while we load your content...