An intramolecular Heck reaction of enol ethers involving β-alkoxyl elimination followed by the β-hydride elimination process: access to (Z)-ortho-formyl/keto-cinnamates†
Abstract
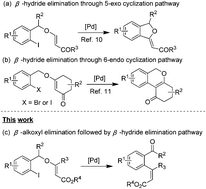
A highly regio- and stereoselective intramolecular Heck reaction of enol ethers involving β-alkoxyl elimination followed by the β-hydride elimination process has been developed. Various o-iodobenzyl enol ethers derived from allenoates or alkynoates are found to be efficient substrates for the syntheses of (Z)-ortho-formyl/keto-cinnamates. It is the first attempt to realize the β-alkoxy elimination of enol ethers in a palladium-catalyzed Heck reaction.


 Please wait while we load your content...
Please wait while we load your content...