Radical alkylation of C(sp3)–H bonds with diacyl peroxides under catalyst-free conditions†
Abstract
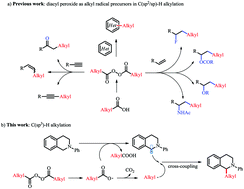
Herein, we describe a protocol for alkylation reactions of C(sp3)–H bonds with diacyl peroxides by means of a process involving cross-coupling between an alkyl radical and an α-aminoalkyl radical. The mild, catalyst- and additive-free conditions make this protocol superior to previously reported C(sp3)–H alkylation strategies. The protocol was applied to 1,2,3,4-tetrahydroisoquinolines and a tetrahydro-β-carboline derivative and could be carried out on a gram scale, indicating its utility for the alkylation of late-stage synthetic intermediates.


 Please wait while we load your content...
Please wait while we load your content...