Quantum chemical prediction of a superelectrophilic dianion and its binding with noble gas atoms†
Abstract
The present communication shows an unprecedented superelectrophilic behaviour of dianion [BeB11(CN)11]2−, containing a positively charged electrophilic center embedded in a negatively charged framework. This dianion is shown to form stable [NgBeB11(CN)11]2− (Ng = He, Ne, Ar, Kr and Xe) compounds associated with either a Ng–Be or Ng–B bond. The dianion [BeB11(CN)11]2− binds covalently with Ar, Kr and Xe via its uncoordinated B atom.
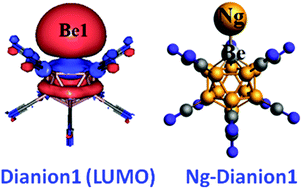


 Please wait while we load your content...
Please wait while we load your content...