One-pot, two-step synthesis of unnatural α-amino acids involving the exhaustive aerobic oxidation of 1,2-diols†
Abstract
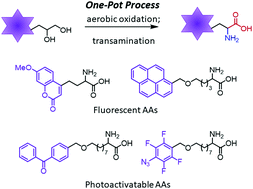
Herein, we report the nor-AZADO-catalyzed exhaustive aerobic oxidations of 1,2-diols to α-keto acids. Combining oxidation with transamination using DL-2-phenylglycine led to the synthesis of free α-amino acids (AAs) in one pot. This method enables the rapid and flexible preparation of a variety of valuable unnatural AAs, such as fluorescent AAs, photoactivatable AAs, and other functional AAs for bioorthogonal reactions.


 Please wait while we load your content...
Please wait while we load your content...