Metal-free α-arylation of α-fluoro-α-nitroacetamides employing diaryliodonium salts†
Abstract
Herein, we present a mild and efficient metal-free arylation of α-fluoro-α-nitroacetamides employing diaryliodonium salts. A broad range of diaryliodonium salts and α-fluoro-α-nitroacetamides containing sensitive functional groups was successfully employed in this protocol to yield the arylated products in good yields. The synthetic value of this novel protocol was further highlighted by extending the α-arylation to α-cyano-α-fluoroacetamides.
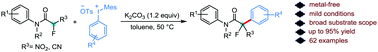


 Please wait while we load your content...
Please wait while we load your content...