An enantioselective oxidative coupling reaction of 2-naphthol derivatives catalyzed by chiral diphosphine oxide–iron(ii) complexes†
Abstract
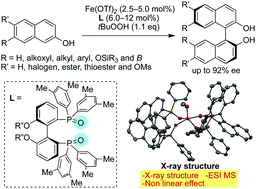
An enantioselective oxidative coupling of 2-naphthol derivatives is developed with the use of chiral Fe(II)–diphosphine oxide complexes. Optically active 1,1-bi-2-naphthol derivatives can be synthesized in high yields when a 2 : 1 complex of (S)-xylyl-iPrO-BIPHEP-oxide and Fe(OTf)2 is used in the presence of t-butyl hydroperoxide as an oxidant. The non-linear effect, X-ray crystal structure and ESI-MS suggest that a 2 : 1 complex of (S)-xylyl-iPrO-BIPHEP-oxide and Fe(OTf)2 is a pre-catalyst for a Fe(III)/Fe(IV) redox cycle.


 Please wait while we load your content...
Please wait while we load your content...