A K-arylacetylide complex for catalytic terminal alkyne functionalization using KOtBu as a precatalyst†
Abstract
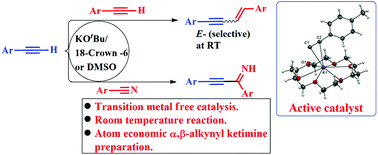
Herein we report a transition metal free catalytic terminal alkyne functionalization across the C–X triple bond (X = CH and N) with E-selective homo (alkyne–alkyne) and head-to-tail selective hetero (alkyne–nitrile) dimerization. A series of stoichiometric reactions enabled us to crystallize a reactive organometallic intermediate K-arylacetylide complex which was characterized by X-ray crystallography, indicating that an ionic mechanism is operative.


 Please wait while we load your content...
Please wait while we load your content...