Rational engineering of amide synthetase enables bioconversion to diverse xiamenmycin derivatives†
Abstract
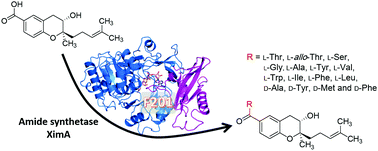
XimA is a unique amide synthetase that belongs to the ANL superfamily of adenylating enzymes, but with a special structural fold. In order to improve the enzyme promiscuity, we engineered XimA by site-directed mutagenesis at a specific position based on our theoretical model of XimA. Thus, we were able to produce diverse benzopyran derivatives with up to 15 different L-form and D-form amino acid substitutions, catalyzed by several XimA variants. Molecular docking and molecular dynamics simulations conducted for various XimA systems provide further structural insights into the substitution effects of the phenylalanine-201 as an active site residue on protein dynamics and enzyme catalysis.


 Please wait while we load your content...
Please wait while we load your content...