An unexpected coupling–reduction tandem reaction for the synthesis of alkenyl-substituted BODIPYs†
Abstract
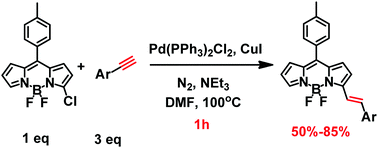
We report an unexpected coupling–reduction tandem reaction as a general and efficient one-pot synthesis of alkenyl-substituted boron dipyrromethene (BODIPY) from chlorinated-BODIPY and alkyne. This unique synthesis combined the Sonogashira coupling reaction and reduction reaction without adding an additional reagent, which shows higher yields, broader substrate scope and faster reaction rate compared with the conventional methods of the Knoevenagel reaction and Heck coupling reaction.


 Please wait while we load your content...
Please wait while we load your content...