Anion ligand promoted selective C–F bond reductive elimination enables C(sp2)–H fluorination†
Abstract
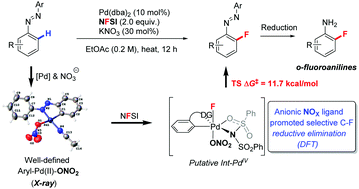
A detailed mechanism study on the anion ligand promoted selective C–H bond fluorination is reported. The role of the anion ligand has been clarified by experimental evidence and DFT calculations. Moreover, the nitrate promoted C–F bond reductive elimination enabled a selective C–H bond fluorination of various symmetric and asymmetric azobenzenes to access diverse o-fluoroanilines.


 Please wait while we load your content...
Please wait while we load your content...