Nickel-catalyzed reductive defunctionalization of esters in the absence of an external reductant: activation of C–O bonds†
Abstract
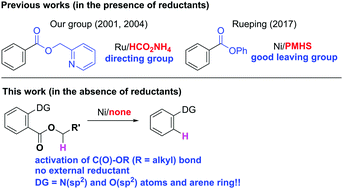
The nickel-catalyzed reductive cleavage of esters in the absence of an external reductant, which involves the cleavage of an inert acyl C–O bond in O-alkyl esters is reported. Various groups, such as N-containing heterocycles, esters, amides, and even arene rings can function as a directing group.


 Please wait while we load your content...
Please wait while we load your content...