Visible-light-promoted oxidative halogenation of alkynes†
Abstract
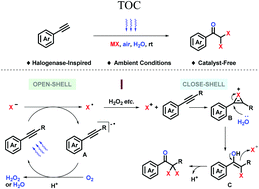
In nature, halogenation promotes the biological activity of secondary metabolites, especially geminal dihalogenation. Related natural molecules have been studied for decades. In recent years, their diversified vital activities have been explored for treating various diseases, which call for efficient and divergent synthetic strategies to facilitate drug discovery. Here we report a catalyst-free oxidative halogenation achieved under ambient conditions (halide ion, air, water, visible light, room temperature, and normal pressure). Constitutionally, electron transfer between the oxygen and halide ion is shuttled via simple conjugated molecules, in which phenylacetylene works as both reactant and catalyst. Synthetically, it provides a highly compatible late-stage transformation strategy to build up dihaloacetophenones (DHAPs).


 Please wait while we load your content...
Please wait while we load your content...