The photoredox alkylarylation of styrenes with alkyl N-hydroxyphthalimide esters and arenes involving C–H functionalization†
Abstract
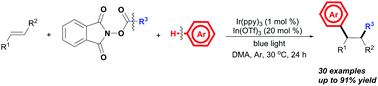
The In(OTf)3-promoted three-component photoredox alkylarylation of styrenes with alkyl NHP esters and arenes to access alkylated arene derivatives through C–C bond cleavage and C–H functionalization is reported. By utilizing visible-light photoredox catalysis, alkyl N-hydroxyphthalimide esters serving as alkyl carbon-centered radicals and a wide range of arenes (e.g., indoles, pyrrole, and electron-rich arenes) as nucleophiles were used to enable the introduction of various alkyl groups and aryl groups across the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds with excellent selectivity and functional group tolerance.
C bonds with excellent selectivity and functional group tolerance.


 Please wait while we load your content...
Please wait while we load your content...