Synthesis of C-terminal glycopeptides via oxime resin aminolysis†
Abstract
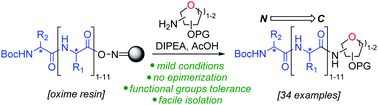
Natural glycopeptides have been shown to possess interesting biological activities. In this work, we have developed a general solid-phase approach to C-terminal glycopeptides. Taking advantage of oxime resin ester bond nucleophile susceptibility, we optimised the nucleophilic cleavage step with glycosylamines and demonstrated the generality and scope of this method. In addition, this reaction has high functional group tolerance and can be used for the preparation of longer C-terminal glycopeptides, demonstrated with the synthesis of a glycododecapeptide in one single step. The results pave the way to access efficiently novel medically relevant compounds.


 Please wait while we load your content...
Please wait while we load your content...