Iron carbonate hydroxide templated binary metal–organic frameworks for highly efficient electrochemical water oxidation†
Abstract
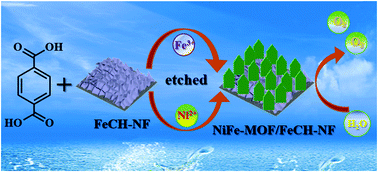
Metal–organic frameworks (MOFs) are promising catalysts for electrochemical reactions. Herein, self-supported NiFe-MOF nanoplates grown on Ni foam (NF) were prepared with iron carbonate hydroxide nanosheets (FeCH NSs) as a semisacrificial template and evaluated for the electrocatalytic oxygen evolution reaction (OER). In this approach, the porous FeCH NSs not only serve as the iron source of NiFe-MOF, but also slow down the leaching of Ni ions from the substrate, thus playing a unique role in regulating the morphology of NiFe-MOF with reduced thickness and sizes, enabling rapid electron transfer and mass transport. The resultant NiFe-MOF/FeCH-NF electrode showed higher activity than FeCH template-free electrodes and superior OER performance over other MOF based binder-free OER electrodes. A current density of 10 mA cm−2 was obtained at a low overpotential of 200 mV with excellent durability in alkaline solution. Raman and TEM measurements reveal the partial transformation of NiFe-MOF to hydroxide during water oxidation.


 Please wait while we load your content...
Please wait while we load your content...