Phosphine-catalyzed (3+2)/(2+3) sequential annulation involving a triple nucleophilic addition reaction of γ-vinyl allenoates†‡
Abstract
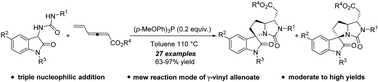
A phosphine-catalyzed (3+2)/(2+3) sequential annulation involving a triple nucleophilic addition reaction of γ-vinyl allenoates was successfully developed. The reaction provided efficient and more practical access to functionalized hydropyrroloimidazolones with good to excellent yields under mild reaction conditions. Notably, γ-vinyl allenoate served as a triple-electrophilic intermediate in this protocol.


 Please wait while we load your content...
Please wait while we load your content...