Effector enhanced enantioselective hydroformylation†
Abstract
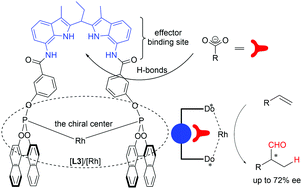
In this communication, we report rhodium DIMPhos complexes with an integrated DIM-receptor that can bind carboxylate containing effectors and their application in the rhodium catalyzed hydroformylation reaction. The binding of chiral effectors in non-chiral [Rh(DIMPhos)] catalysts does not lead to enantioselective hydroformylation, but the binding of either achiral or chiral effectors can significantly enhance the enantioselectivity induced by the chiral Rh-metal complexes. For example, the supramolecular complex [Rh]/[1S⊂L3] displays high regio- and enantioselectivity in the hydroformylation of vinyl acetate (72% ee, and b/l >99), whereas in absence of this effector the ee is around 17%.


 Please wait while we load your content...
Please wait while we load your content...