Formation of an NHC-stabilized heterocyclic housane and its isomerization into a cyclopentenyl anion analogue†
Abstract
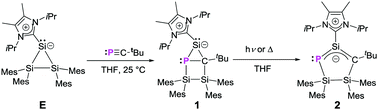
Addition of :P![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–tBu to the NHC-coordinated trisilacyclopropylidene Si3Mes4NHCiPr2Me2 afforded a heavier analogue of a bicyclo[2.1.0]pentane, a so-called “housane”, that rearranges under irradiation with a high-pressure mercury lamp or at 60 °C to an NHC-stabilized cyclopentenyl anion isomer with a three-center four-electron (3c-4e) π-bond.
C–tBu to the NHC-coordinated trisilacyclopropylidene Si3Mes4NHCiPr2Me2 afforded a heavier analogue of a bicyclo[2.1.0]pentane, a so-called “housane”, that rearranges under irradiation with a high-pressure mercury lamp or at 60 °C to an NHC-stabilized cyclopentenyl anion isomer with a three-center four-electron (3c-4e) π-bond.


 Please wait while we load your content...
Please wait while we load your content...