Suppressing lithium dendrite formation by slowing its desolvation kinetics†
Abstract
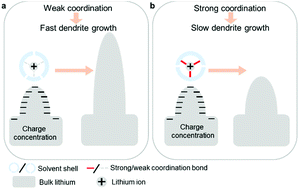
Slowing the dendrite formation process is one way to alleviate the fast capacity fade and safety issues in lithium metal battery systems. We used tetraethylene glycol dimethyl ether (TEGDME) as a complementary solvent to increase the desolvation activation energy of Li+, reduce the speed of lithium electrodeposition kinetics, and suppress dendrite formation. Density functional theory calculations combined with Raman spectroscopy indicate that a stronger coordination interaction is obtained between Li+ and TEGDME than between Li+ and 1,2-dimethoxyethane (DME) or 1,3-dioxolane (DOL). Such a strong coordination leads to a slower electrochemical reaction rate. As a result, uniform lithium electrodeposition morphology and good cycling stability of a Li|Li symmetric cell for more than 500 hours were achieved. Our approach suggests a way in which dendrite formation can be controlled by the electrochemical reaction itself.


 Please wait while we load your content...
Please wait while we load your content...