A ZnI2-catalyzed regioselective cascade 1,4-conjugate addition/5-exo-dig annulation pathway for one-pot access to heterobiaryl frameworks†
Abstract
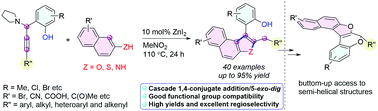
Facile access to π-extended heterobiaryl compounds via a non-cross-coupling strategy has been achieved. In the presence of an inexpensive ZnI2 catalyst and versatile propargylamine and β-naphthol (or β-naphthylamine and β-naphthyl mercaptan) starting materials, a variety of sterically hindered heterobiaryl frameworks can be easily obtained. The present catalytic system offers excellent selectivity, good-to-excellent product yields, and good functional group tolerance including, for instance, –CN, –COOH, –C(O)R, –Br and –Cl groups. This cyclization process is proposed to proceed via in situ generated alkynyl O-quinone methides (O-AQMs), following a cascade intermolecular 1,4-conjugate addition/5-exo-dig annulation/1,3-H shift pathway.


 Please wait while we load your content...
Please wait while we load your content...