Divergent synthesis of N-heterocyclic 1,6-enynes through a zinc-catalyzed decarboxylative A3 reaction†
Abstract
A zinc-catalyzed decarboxylative A3 reaction of cyclic amino acids, α,β-unsaturated aldehydes and terminal alkynes has been developed. A series of functionalized N-heterocyclic 1,6-enynes have been successfully obtained with excellent regioselectivities through this novel approach. In addition, the utility of this straightforward process is demonstrated by the preparation of a polycyclic nitrogen-containing heterocyclic compound.
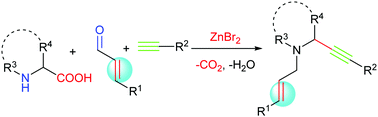


 Please wait while we load your content...
Please wait while we load your content...