Unravelling the mechanism of amyloid-β peptide oligomerization and fibrillation at chiral interfaces†
Abstract
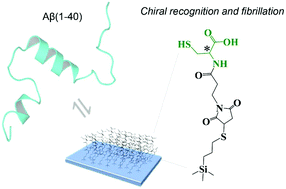
In this communication, the mechanism of how surface chirality affects amyloid-β (Aβ) fibrillation was firstly unravelled at the molecular level: a chiral surface serves to control the 2D-diffusion and surface residence time of Aβ molecules via the chiral recognition with Aβ to allow precursor Aβ to laterally diffuse and collide with each other for oligomerization and fibrillation. Surface chirality that shortens the surface residence time of Aβ, for example, R-cysteine modification with carboxylic, secondary amine and thiol groups surrounding the chiral center, can retard Aβ oligomerization and fibrillation. This work is essential to a deeper fundamental understanding of the effects of surface chirality on amyloidosis processes as well as the development of chiral materials to inhibit Aβ fibrillation.


 Please wait while we load your content...
Please wait while we load your content...