Highly chemoselective, sterically sensitive NHC-catalysed amine acylation with pyridil†
Abstract
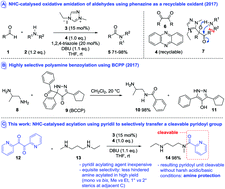
A new strategy for the protection of amines has been developed involving reaction with pyridil under the influence of N-heterocyclic carbene catalysis. The methodology is capable of distinguishing between two amines characterised by small differences in steric bulk and the resulting pyridoyl amides can be cleaved without requiring either strongly acidic or basic hydrolysis.


 Please wait while we load your content...
Please wait while we load your content...