Highly chemoselective difluoromethylative homologation of iso(thio)cyanates: expeditious access to unprecedented α,α-difluoro(thio)amides†
Abstract
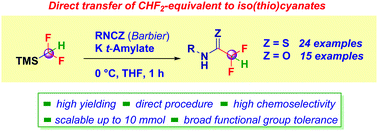
The new motif – α,α-difluoromethyl thioamide – has been assembled starting from isothiocyanate (as thioamide precursor) and a formal difluoromethyl-carbanion generated from commercially available TMSCHF2. Upon proper activation of this reagent with potassium tert-amylate, the high-yielding transfer of the difluorinated nucleophile takes place under high chemocontrol. Various sensitive functionalities (e.g. ester, nitrile, nitro, azido groups) can be accommodated across the isothiocyanate core, thus allowing a wide scope. The methodology is highly flexible and adaptable to prepare analogous α,α-difluoromethyl oxoamides by conveniently using isocyanates as the electrophilic building-blocks.


 Please wait while we load your content...
Please wait while we load your content...